Bolt Carrier Group Coatings and Treatments | Black Rifle Depot
BOLT CARRIER GROUP COATINGS AND TREATMENTS
- Extensive technical data for each coating and treatment.
- Ensuring a thorough understanding of their impact on the BCG's performance.
- Durability.
- Overall effectiveness.
TREATMENTS:
To hyperlink to the section you want - click on the name. NICKEL BORON | BLACK NITRIDE/MELONITE | PARKERIZED |NICKLE TEFLON | TITANIUM NITRIDE | CHROME | CERAMIC | IONBONDNICKEL BORON
Buy a Nickel Boron BCG UCT COATINGS’ EXO® PATENTED NICKEL BORON TECHNOLOGY
UCT's innovative Nickel Boron plating, trademarked as EXO®, represents a significant advancement in surface treatments, offering a unique competitive edge in various applications and industries. The EXO® Process utilizes a Nickel Boron alloy with a higher boron content than any commercially available Ni3B. This careful formulation enhances material properties, such as increased hardness and superior wear resistance. Consequently, parts treated with EXO® Nickel Boron exhibit longer operational life and reduced maintenance needs. Furthermore, this patented coating notably improves heat dissipation, ensuring that treated products operate at cooler temperatures than their non-treated counterparts.
EXO® is more than just a surface layer; it's a patented, nodular Nickel-Boron (Ni3B) surface treatment that fundamentally alters the surface of the substrate metal. Unlike traditional coatings that merely adhere to the surface, the Ni3B in EXO® integrates at the atomic level with the substrate. This deep integration not only enhances the hardness of the surface but also maintains the original properties of the substrate, thereby overcoming the limitations in cost, weight, and degradation typically associated with conventionally hardened metals.
Educational Overview of EXO® Benefits:
UCT COATINGS’ EXO® PATENTED NICKEL BORON TECHNOLOGY
UCT's innovative Nickel Boron plating, trademarked as EXO®, represents a significant advancement in surface treatments, offering a unique competitive edge in various applications and industries. The EXO® Process utilizes a Nickel Boron alloy with a higher boron content than any commercially available Ni3B. This careful formulation enhances material properties, such as increased hardness and superior wear resistance. Consequently, parts treated with EXO® Nickel Boron exhibit longer operational life and reduced maintenance needs. Furthermore, this patented coating notably improves heat dissipation, ensuring that treated products operate at cooler temperatures than their non-treated counterparts.
EXO® is more than just a surface layer; it's a patented, nodular Nickel-Boron (Ni3B) surface treatment that fundamentally alters the surface of the substrate metal. Unlike traditional coatings that merely adhere to the surface, the Ni3B in EXO® integrates at the atomic level with the substrate. This deep integration not only enhances the hardness of the surface but also maintains the original properties of the substrate, thereby overcoming the limitations in cost, weight, and degradation typically associated with conventionally hardened metals.
Educational Overview of EXO® Benefits:
- Hardness: Achieves a Rockwell C rating of over 82, translating from its typical measurement of over 1300 Knoop, indicating significant surface hardness.
- Low Friction Coefficient: Exhibits a typical range of 0.08 to 0.2, contributing to smoother operations and reduced wear.
- Uniformity: The 'non-line-of-sight' application ensures total coverage, even on parts with complex geometries, leading to uniform protection.
- Hard Chrome Replacement: EXO® surpasses hard chrome in hardness and lower friction coefficient while being more environmentally friendly.
- Single-Process Solution: Replaces multiple coating processes, streamlining manufacturing and reducing complexity.
- Technological Advantages: The treatment is repeatable, scalable, and transferable, offering consistent quality across various applications.
- Applicability: Suitable for all steel alloys, broadening its range of use.
- Versatility in Application: Ideal for virtually any context where reduced friction, prolonged service life, enhanced performance, and lower maintenance are sought after.
BLACK NITRIDE/MELONITE
Buy a Black Nitride BCG
Melonite® - QPQ® Thermochemical Quench-Polish-Quench (DHQN)
According to IBC Coatings Technologies, they use a thermochemical quench-polish-quench (QPQ® see registered trademarks disclaimer at the bottom of the page) nitriding process they say is equivalent to Melonite® to strengthen, harden, and improve the overall corrosion resistance of the metal components or equipment. Unlike some other treatments and finishes, Melonite® has a very consistent and predictable outcome that often significantly strengthens the surface. Including surfaces such as carbon steel, stainless steel, alloy steel, and austenitic steel products. It can also be quite effective when used on cast iron as well as sintered iron. Melonite® case hardening involves a QPQ® diffusion process. It creates a highly stable and uniform surface that is significantly harder than untreated metal. Even under the harshest conditions, the parts, components, and equipment treated with this form of nitrocarburizing (liquid nitriding) are far more resistant to normal wear and tear. The process involves a series of steps. They are submersion in molten salt baths, polishing, and rinsing, during which nitrogen, carbon, and oxygen are diffused into the surface of the metal, creating the case hardening effect allowing for extended and longer lasting use. Melonite® processing produces an improved, hardened, corrosion-resistant surface that will out-perform other forms of metal plating (ex: hard chrome, zinc, or nickel), gas nitriding, Parkerizing®, and black oxide or bluing. While these traditional surface finishing processes were used in many industries for many years, they are each being methodically replaced by the superior Melonite® finishing process.How Does QPQ® Work?
The first step (nitriding) in the Melonite® process is to preheat the parts in a furnace. Then after the parts are the appropriate temperature, they are immersed in a molten, aerated solution of salts containing both nitrogen and carbon. This step, can generally last up to two hours, depending on the parts and the conditions they will be exposed to. After nitriding, a surface layer of corrosion resistant magnetite (Fe3O4) is created using an oxidation bath, where the parts remain usually for a period of up to 30 minutes. The parts are then fully quenched and rinsed using a water bath. The next phase in the process is polishing. This phase is specifically used to restore the part’s original surface finish. Polishing techniques and processes vary. But polishing may be accomplished with centerless or vibratory polishing, lapping, glass bead blasting, or by another method of surface polishing. Once the polishing is finished, the part will undergo a similar process again. The part will be immersed in an oxidizing salt bath, a water quench and rinse. The process is referred to as quench-polish-quench, or QPQ®. -In addition to being harder and more wear resistant, when the QPQ® process is complete, the parts will have a smooth black finish that is clean and attractive with no need for painting or further finishing treatments.Advantages of Melonite® Case Hardening:
Consider a few advantages that this process has over other alternatives:- Improved wear and corrosion resistance
- Improved heat and friction resistance
- Increased fatigue strength
- An attractive black finish
- Less friction between moving parts
- Smoother surfaces
 Figure 1: Typical case depths achieved by QPQ® salt bath nitriding on three representative materials, 1018 mild steel, 4140 alloy steel, and H13 tool steel.Figure 2: Cross section of 1018 steel after QPQ® salt bath nitriding, showing the hard and wear resistant compound layer of iron nitride formed at the surface.
Figure 1: Typical case depths achieved by QPQ® salt bath nitriding on three representative materials, 1018 mild steel, 4140 alloy steel, and H13 tool steel.Figure 2: Cross section of 1018 steel after QPQ® salt bath nitriding, showing the hard and wear resistant compound layer of iron nitride formed at the surface.
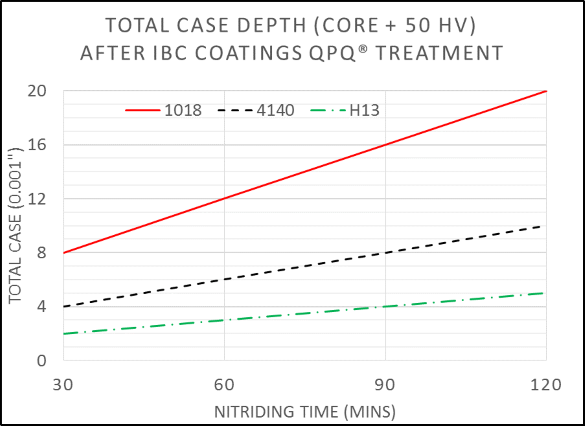 Table 1: Typical surface and core hardness results after QPQ® salt bath nitriding
Table 1: Typical surface and core hardness results after QPQ® salt bath nitriding
|
MATERIAL |
SURFACE HV1KG |
SURFACE HV10KG |
CORE HV1KG |
| 1018 mild steel | 400 | 300 | 200 |
| 4140 alloy steel | 800 | 650 | 350 |
| 416 stainless steel | 1000 | 600 | 300 |
| H13 tool steel | 1200 | 750 | 500 |
PARKERIZED
Buy a Parkerized BCG
Parkerization (Zink/Manganese Phosphate)
Parkerizing is a phosphate coating is used on steel parts for corrosion resistance, lubrication, or as a foundation for subsequent coatings or painting. It serves as a conversion coating in which a dilute solution of phosphoric acid and phosphate salts is applied via spraying or immersion and chemically reacts with the surface of the part being coated to form a layer of insoluble, crystalline phosphates. Phosphate conversion coatings can also be used on aluminum, zinc, cadmium, silver, and tin.
The main types of phosphate coatings are manganese, iron, and zinc. Manganese phosphate are used both for corrosion resistance and lubricity and are applied only by immersion. Iron phosphates are typically used as a base for further coatings or painting and are applied by immersion or by spraying. Zinc phosphates are used for corrosion resistance (phosphate and oil), a lubricant base layer, and as a paint/coating base and can also be applied by immersion or spraying.
Process
- cleaning the surface
- rinsing
- surface activation
- phosphating
- rinsing
- neutralizing rinse (optional)
- drying
- application of supplemental coatings: lubricants, sealers, oil, etc.
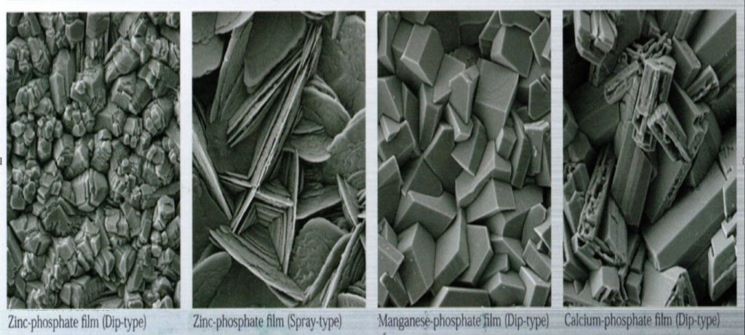 The performance of the phosphate coating is significantly dependent on the crystal structure as well as the weight. For example, a micro-crystalline structure is usually optimal for corrosion resistance or subsequent painting. A coarse grain structure impregnated with oil, however, may be the most desirable for wear resistance. These factors are controlled by selecting the appropriate phosphate solution, using various additives, and controlling bath temperature, concentration, and phosphating time. A widely used additive is to seed the metal surface with tiny particles of titanium salts by adding these to the rinse bath preceding the phosphating. This is known as activation.
The performance of the phosphate coating is significantly dependent on the crystal structure as well as the weight. For example, a micro-crystalline structure is usually optimal for corrosion resistance or subsequent painting. A coarse grain structure impregnated with oil, however, may be the most desirable for wear resistance. These factors are controlled by selecting the appropriate phosphate solution, using various additives, and controlling bath temperature, concentration, and phosphating time. A widely used additive is to seed the metal surface with tiny particles of titanium salts by adding these to the rinse bath preceding the phosphating. This is known as activation.
NICKEL TEFLON
Electroless Nickel PTFE (Teflon®)
The defense industry use electroless nickel-PTFE when components require more lubricity than traditional plating surfaces. EN-PTFE is hard, and ideal for sliding and wear applications. It is also dry lubricating, highly lubricious, non-wetting, and has excellent wear and corrosion resistance. EN-PTFE is a low friction wear plating. It is also now used as a more environmentally friendly alternative to cadmium-plated connectors.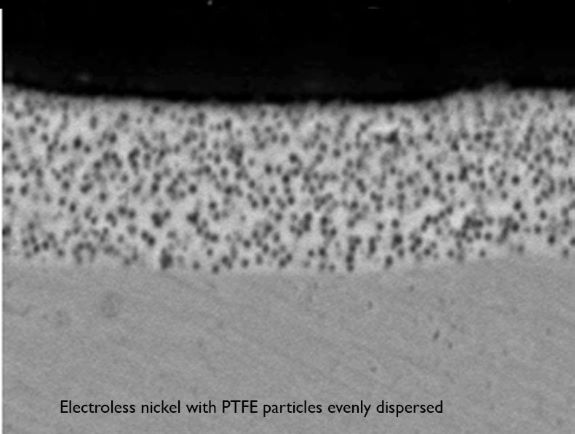
Teflon Particles Co-Deposited in Electroless Nickel Increase Lubricity
- Extreme self-lubrication (due to PTFE)
- Superior wear, corrosion, and friction resistance
- Dry lubrication
- Low coefficient of friction (vital for sliding, moving & friction-wear applications)
- Enhanced release, non-stick surface (for molding operations)
- Uniform coating and deposit thickness (even on complex geometries)
- Non-wetting surface repels water, oil, and other potential contaminants (due to PTFE)
- Hardness:Phosphorus Composition: 7-10% by weight
- 250 – 300 HK100 (as plated)
- 375 – 425 HK100 (heat treated, 350°C/2 hours)
- PTFE (Teflon®) Composition: 4-9% by weight (13%-27% by vol.)
- Typical coating thickness of .0001″ – .0005″ thick
- Coefficient of Friction: Less than 0.10
- End-Of-Life Vehicle (ELV) Regulations and Restriction of Certain Hazardous Substances (RoHS) compliant
- Conforms to SAE 2454 specifications
- Ideal in low loading and low temperature applications
- Repels water, oil, and other potential contaminants (due to PTFE)
TITANIUM NITRIDE

Description
Titanium Nitride (TiN) the staple of thin film coating solves tribological problems with machine components that can be coated at temperatures of 425°C - 450°C. TiN is normally applied to steels, hardened steels, and stainless steel materials where high wear resistance and lubricity is needed. TiN is the staple of Physical Vapor Deposition coatings ideally suited for applications that use expensive tooling such as injection molding, sawing, tapping, and forming. TiN can be easily stripped making recoating an option. Can be stripped and recoated.Substrate(s)
- Steel
- Hardened Steel
- Titanium
- Stainless Steel
Temp. Ratings
- Process Temp: 840°(448°C)
- Max Temp: 1110°(598°C)
Thickness
- Thickness (micron): 1 - 7
Color(s)
- Gold
Process
TiN is applied using lateral rotating cathode technology in a PVD (physical vapor deposition) process. Material is vaporized from a solid source in the form of atoms/molecules and then transported in the form of a vapor through a vacuum, low pressure gas/plasma to the substrate where it condenses.Advantages
High hardness, Abrasive wear resistance, higher reliability in dry operations, Lubricates can be reduced or replaced, Increased tool life- Increased Hardness
- Increased Lubricity
- Low Coefficient of Friction
- Wear Resistant
Figure 1
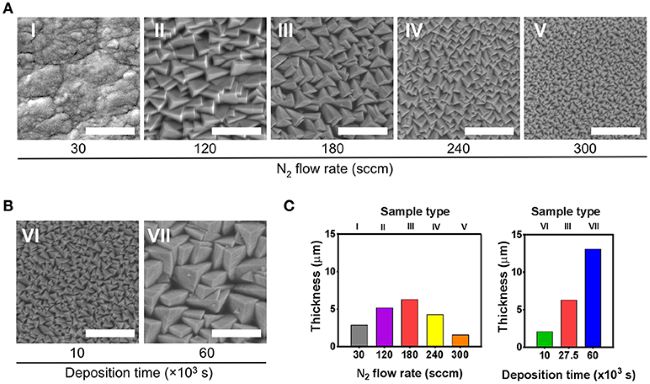 Figure 1. Scanning electron microscopy (SEM) analysis of the seven types of prepared TiN films (I–VII). (A) Effect of varying the N2 flow rate on film morphology. For this set of samples (I–V), the deposition time was kept at 27.5 × 103 s. Films type I were used as smooth reference coatings. (B) Effect of varying the deposition time on film morphology, while keeping the N2 flow rate at 180 sccm. (C) Thickness of the films displayed in (A,B). Scale bar in SEM images represents 1 μm.
Figure 1. Scanning electron microscopy (SEM) analysis of the seven types of prepared TiN films (I–VII). (A) Effect of varying the N2 flow rate on film morphology. For this set of samples (I–V), the deposition time was kept at 27.5 × 103 s. Films type I were used as smooth reference coatings. (B) Effect of varying the deposition time on film morphology, while keeping the N2 flow rate at 180 sccm. (C) Thickness of the films displayed in (A,B). Scale bar in SEM images represents 1 μm.
Figure 2
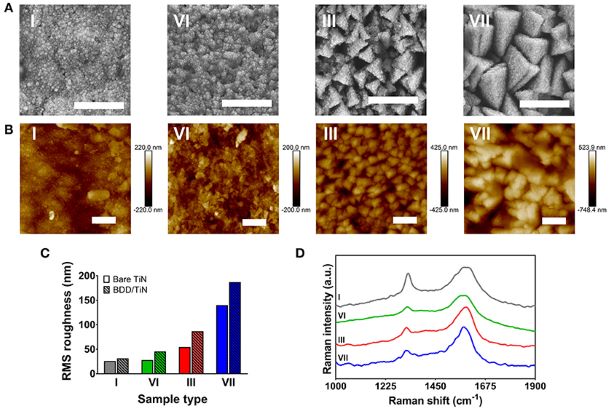 Figure 2. Surface analysis of four representative electrodes (type I, III, VI, and VII). (A) Scanning electron microscopy (SEM) images displaying the uniform coverage of the nanocrystalline diamond layer. Scale bar represents 1 μm. (B) Atomic force microscopy (AFM) height images. Scale bar represents 1 μm. (C) AFM surface roughness measurements from the films displayed in (B) before (bare TiN) and after diamond deposition (BDD/TiN). (D) 325-nm Raman spectra showing the diamond-related peak between 1,320 and 1,328 cm−1. The spectra have been offset for clarity.
Figure 2. Surface analysis of four representative electrodes (type I, III, VI, and VII). (A) Scanning electron microscopy (SEM) images displaying the uniform coverage of the nanocrystalline diamond layer. Scale bar represents 1 μm. (B) Atomic force microscopy (AFM) height images. Scale bar represents 1 μm. (C) AFM surface roughness measurements from the films displayed in (B) before (bare TiN) and after diamond deposition (BDD/TiN). (D) 325-nm Raman spectra showing the diamond-related peak between 1,320 and 1,328 cm−1. The spectra have been offset for clarity.
CHROME

Hard Chrome Plating
Electroplating requires the metal piece intended for plating to be connected to the negative terminal (cathode) of a DC current power source. The metal that will form the coating is connected to the positive terminal (anode) of the power source.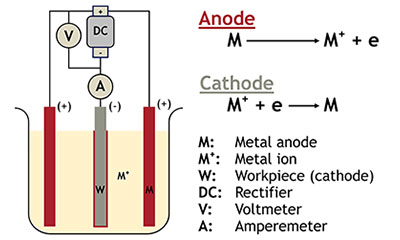 Both are placed in an electrolyte solution that contains one or more dissolved metal salts that permit the flow of electricity. Metal from the positive terminal end (the anode) dissolves in the electrolyte and then deposits on the metal connected to the negative terminal (the cathode)*.
*In some cases, the anode is not actually consumed in the process, but rather, the metal salts dissolved in the electrolyte are deposited on the cathode.
When electroplating, the cathode is generally placed in the middle between two anodes, so that the deposits are made evenly on both sides of the part.
Hard Chrome Plating. It was invented in the 1920s and perfected in the 1940-1950s. In ordinary chrome plating, which is used for decorative purposes, the coating is very thin and beautiful, whereas hard chrome plating is not as beautiful, but is a much thicker surface. Hard Chrome Plating is also called Engineering Chrome Plating, as it is very much used in surfaces that need a good corrosion resistance as well as lubrication. This type of plating is much harder than nickel plating. It rates between 65-70 HRC on the Rockwell Hardness scale, which puts it harder than high-quality steel alloys such as custom knife edges. They can also show superior resistance to rusting in punishing environments such as salt spray. Chrome plated firearms generally come in bright or matte finishes.
There is also another chrome plated finish called Black Chrome Plating, where the steel firearm is initially nickel plated, and then chrome plated on top of the nickel plating. It is a softer finish than hard chrome plating, but some people believe the distinct finish is worth it.
Both are placed in an electrolyte solution that contains one or more dissolved metal salts that permit the flow of electricity. Metal from the positive terminal end (the anode) dissolves in the electrolyte and then deposits on the metal connected to the negative terminal (the cathode)*.
*In some cases, the anode is not actually consumed in the process, but rather, the metal salts dissolved in the electrolyte are deposited on the cathode.
When electroplating, the cathode is generally placed in the middle between two anodes, so that the deposits are made evenly on both sides of the part.
Hard Chrome Plating. It was invented in the 1920s and perfected in the 1940-1950s. In ordinary chrome plating, which is used for decorative purposes, the coating is very thin and beautiful, whereas hard chrome plating is not as beautiful, but is a much thicker surface. Hard Chrome Plating is also called Engineering Chrome Plating, as it is very much used in surfaces that need a good corrosion resistance as well as lubrication. This type of plating is much harder than nickel plating. It rates between 65-70 HRC on the Rockwell Hardness scale, which puts it harder than high-quality steel alloys such as custom knife edges. They can also show superior resistance to rusting in punishing environments such as salt spray. Chrome plated firearms generally come in bright or matte finishes.
There is also another chrome plated finish called Black Chrome Plating, where the steel firearm is initially nickel plated, and then chrome plated on top of the nickel plating. It is a softer finish than hard chrome plating, but some people believe the distinct finish is worth it.
Process
This is generally the process used when plating a weapon:- The surfaces are first polished to as smooth as possible, because small bumps will show up even more after the surfaces are plated.
- The parts are cleaned in detergent to remove all traces of grease and oil
- The parts are then rinsed in water to remove all traces of detergent.
- In case of electroless process, the parts are coated with the catalyst
- The parts are placed in the electrolyte solution or electroless solution and the solution is agitated gently with air bubbles to prevent formation of gas bubbles sticking to the part. Gas bubbles sticking to the surface prevents metal from depositing there, so it is important to get rid of them.
- The parts are taken out and dried and oiled.
- The parts are then reassembled.
CERAMIC

Thin-Film Ceramic Coating?
Thin film Ceramic Coatings is a generic term for a modern protective coating made from micro and nano scale ceramic particles that are mixed with a polymer binder. When the ceramic protective coating is appropriately selected based on the substrate and intended use, a dramatic increase in useful life of the component can be achieved due to improved abrasion, corrosion, oxidation, chemical and UV resistance. Ceramic coating products are offered from multiple high-quality manufacturers such as Cerakote, Saint Gobain, Techline, and others.What is Cerakote (Ceramic Coating)?
Cerakote is a top-quality Polymer-Ceramic coating that can be applied to metals, plastics, polymers, and wood. The unique formulation of Cerakote ceramic coatings enhances several physical performance properties including abrasion/wear resistance, corrosion, oxidation resistance, chemical resistance, and even in some cases UV resistance. Cerakote also features high impact strength, hardness, thermal barrier properties, and more. It is a very versatile coating available in hundreds of colors to improve aesthetics as well as enhance protection against environmental damage. How durable is Ceramic Coatings (Cerakote)? ASTM D2794) Cerakote has the highest (reasonably measurable) drop test result. The toughness implied by the drop test defers to the lamination and deformation resistance of a coating. Cerakote gun coatings achieve the 160 inch-lb maximum meeting or exceeding all other competing products. (ASTM B117) Cerakote shines in the corrosion resistance testing. It exceeds all relative competitors in gun coatings and gun finishes in the salt spray testing which determines how easily a piece of material is oxidized by “normal” and slightly elevated environmental exposure. (ASTM D4060) Cerakote has abrasion resistance far superior to ANY other standard gun coatings or gun finishes. The friction testing helps to determine the wear capabilities of the product, and in these tests, Cerakote as a gun coating excels.IONBOND

Ionbond Diamond like Carbon (DLC)
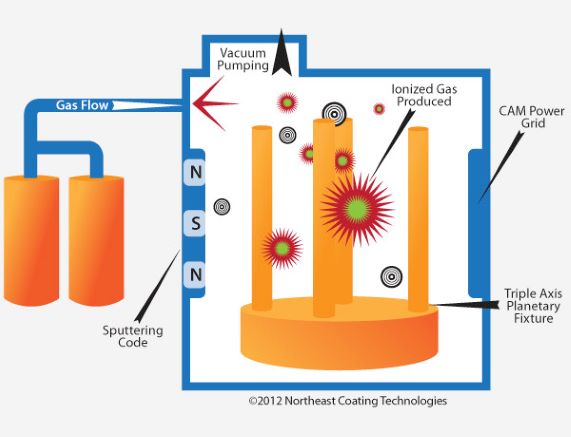
DLC is a great coating for ferrous alloys (8620, 9310, c158 etc..) and stainless steels (1740, 415R and 416R etc..). It is known as the plasma assisted vapor deposition coating called Tungsten Diamond Like Carbon. One outstanding feature of the Diamond-Like Coating in that the parts are never heated over 300 degrees Fahrenheit, this means there is no opportunity for the parts to lose their temper, soften, or become brittle. Typically, two microns of the material is deposited on the surface during the DLC Process. 2 microns amounts to about 80 millionths of an inch, so there is no effect on any of the parts' function or fit. DLC is a thoroughly impressive treatment. Not only does it perform its intended task of coloring the bright steel to a matte, non-reflective greyish black, it also provides a tough, corrosion-proof coating that is virtually scratch proof. DLC will not wear even when subjected to extensive use.
Quality DLC Deposition
DLC coatings can be deposited using a variety of deposition methods such as sputtering, ion beam, cathodic arc, electron beam, lasers and PACVD. Some companies deposit the diamond like carbon coatings using a patented technology combining PVD (Physical Vapor Deposition) and PACVD/Ionbond (Plasma Assisted Chemical Vapor Deposition) which deposits some of the highest quality, most consistent DLC coatings available to industry today. This technology allows the DLC coatings to be deposited in large production machines without sacrificing film quality.